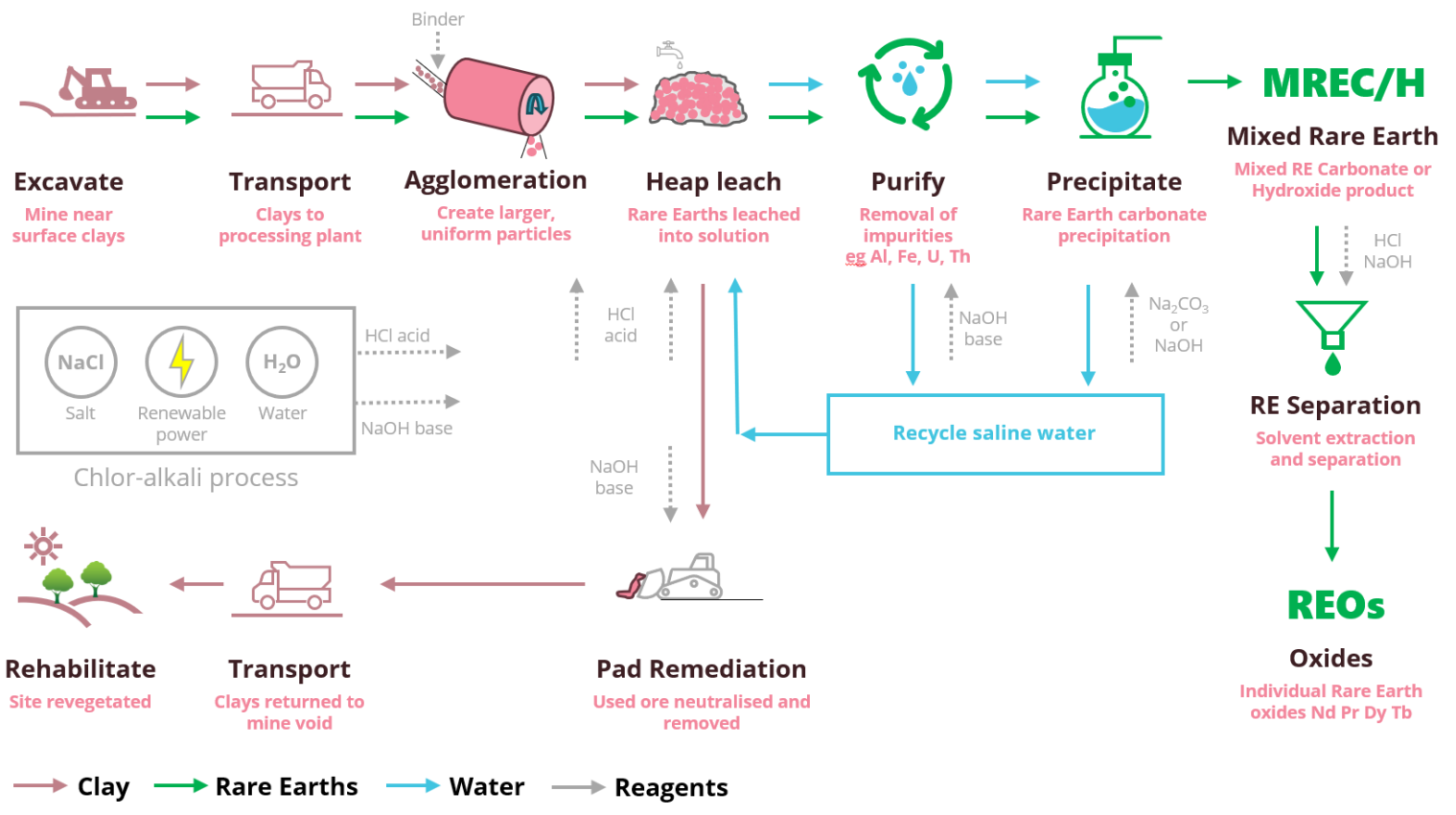
Indicative Processing Steps
OD6 metals is proposing the following simplified processing map to deliver rare earth products based on the test work completed to date.
Central to the flowsheet is the use of a site based chlor-alkali facility that utilises salt and water to produce two reagents, namely hydrochloric acid (HCl) and sodium hydroxide (NaOH). HCl is used to leach the rare earth elements, then NaOH is used to remove impurities, precipitate a mixed rare earth carbonate or hydroxide product (MREC/H) and neutralise the clays prior to in pit disposal.
Chlor-alkali Process and Vendor Information
The chlor-alkali process involves passing an electric current through high purity sodium chloride (NaCl or salt) brine to produce hydrogen, chlorine and caustic soda. The hydrogen and chlorine can then be combined into hydrochloric acid. The equations to create caustic soda (NaOH) and hydrogen chloride (HCl) are:
2H2O (l) + 2NaCl (aq) —-> H2 (g) + Cl2 (g) + 2NaOH (aq)
H2 (g) + Cl2 (g) —–> 2HCl (g)
OD6 has obtained information from and held discussions with chlor-alkali electrolyser vendors and experts to determine if owning and operating a site based facility is a viable option. To date all indications are that this is likely to provide the lowest operating cost for a long term project that will form an integrated processing facility with storage tanks for HCl and NaOH to buffer any disruptions.
Based on the publicly available information associated with a BICHLOR™ Electrolyser, plus informal discussion, the following key details are noted for a single chlor-alkali electrolyser:
- Power consumption is the major cost at 1,990 kWhr/te NaOH @ 6 kA/m2.
- Normally allow for 350 days operation and 7kA/m2.
- Normally operate at 385mbarg, 90°C and 32wt% NaOH.
- Can produce up to 62ktpa HCl and 69ktpa of NaOH.
- Indicative pricing for a chlor-alkali electrolyser is approximately £3M each (A$5.7M).
- Multiple smaller electrolysers can be utilised to provide operation flexibility.
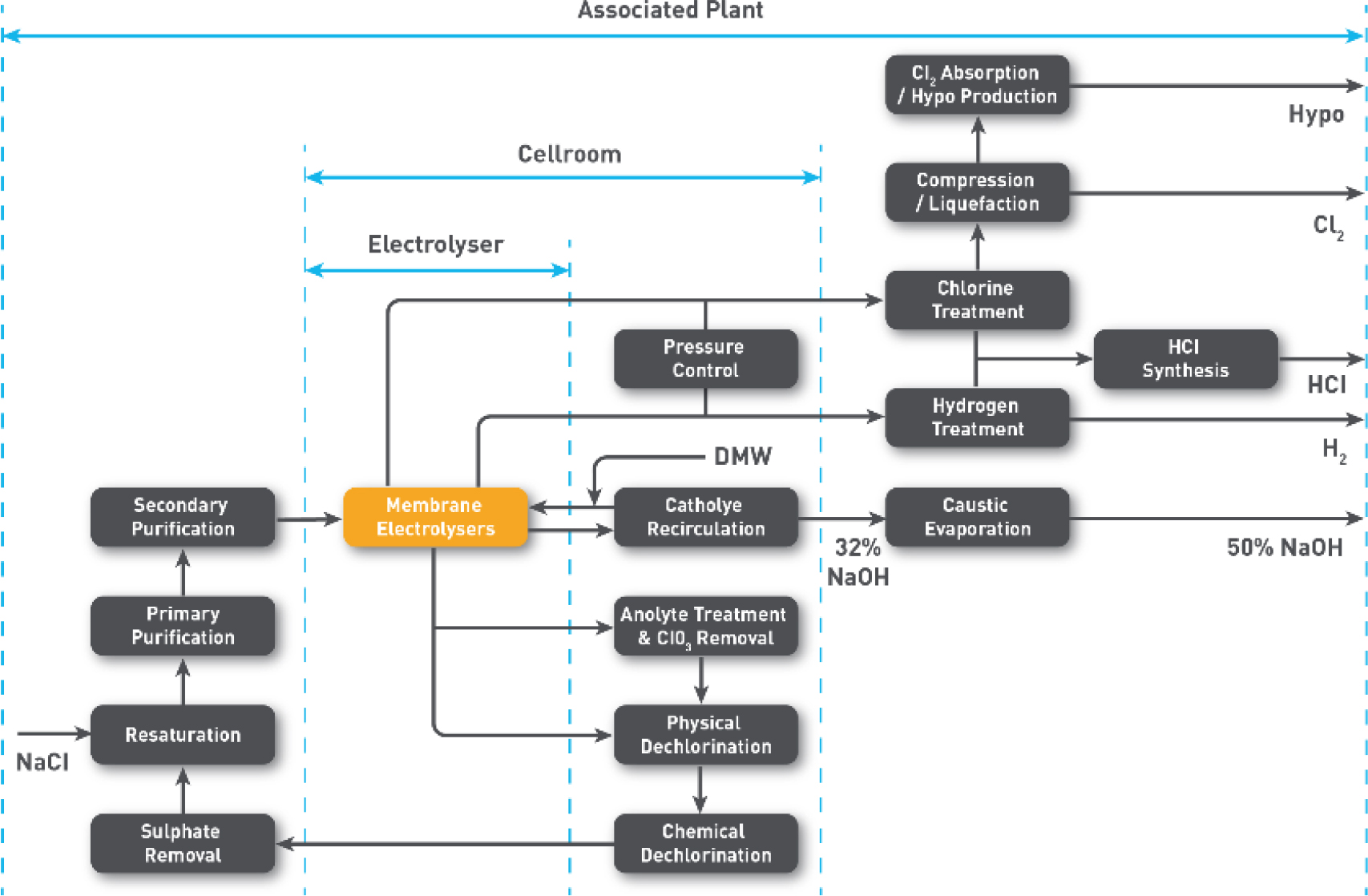
A chlor-alkali facility would also consist of the key production steps outlined in Figure 2 along with the associated equipment. Figure 3 also provides an example of scale of two operational
electrolysers.
At an average consumption of 16 kg HCl / tonne of ore a single electrolyser based on the above information could provide sufficient reagents to treat ~4Mtpa of clay ore. The average acid consumption may potentially be lower based on the potential removal of coarse grained (>75um) material which is yet to be reported.
Given power being the main cost driver OD6 envisages that low-cost power supply would be sourced from an owned and operated hybrid power system consisting of solar, wind turbines, energy storage and gas or diesel powered generators. OD6 understands that a similar facility that is currently powering the Esperance township and surrounding areas is currently achieving a 70% renewable power penetration rate which is significant achievement that OD6 should aim to replicate.
On an owned and operated basis, utilising low cost power supply, OD6 has an aspiration target to achieve an operational reagent consumption cost that would be equivalent to about $5-6/t of processed clay ore, assuming sunk capital costs.